The Tiling Tree Method, Part 2: Common Pitfalls and How to Overcome Them
How to *really* think of every way of solving a problem
Thinking of every possible solution for a given problem can be a tricky task. How can we possibly catalogue all of the solutions that could be done - and then, to choose the optimal path, perhaps one that has never been thought of before?
We described the Tiling Tree Method1, which enables you to do just this, in an earlier article. In summary, this method requires one to perform iterative splits on a solution space, dividing a set of possible solutions into increasingly smaller subsets. The resulting diagram looks like a tree. The subsets carved from a parent set should “tile” the space of possibilities - that is, one subset should not overlap with another subset, and the subsets, taken collectively, should not leave out any possibility. Ultimately, you might arrive at individual ideas, the “leaves” of the tree, which then could be evaluated for their impact, uniqueness, and feasibility - or whatever criteria are at hand. (See the aforementioned article for examples, and general guidelines.)
This strategy may appear simple to execute, yet to do it well is quite nuanced, since there are many ways to do it wrong (though practice, as with any skill, is extremely helpful). We (Claire and Nina) are newer to the art than Ed, and for the purposes of this blog and our own learning, decided to create a tree to help us think of novel ways to deliver genes into the body - a big need in basic biology research, and also in therapeutic treatment of diseases. Delivery vehicles for genetic material could be very useful for gene therapy, for example. We wanted to interact, as we generated the trees, as researchers might while working at the bench in the lab, getting feedback from Ed along the way.
We went through multiple iterations and learned a lot each time. Importantly, the changes we made over the iterations, addressed mistakes that people commonly make when generating such trees. Thus, our example may help you avoid pitfalls, and make trees that truly tile—helpful for generating novel ideas.
Our first tiling tree
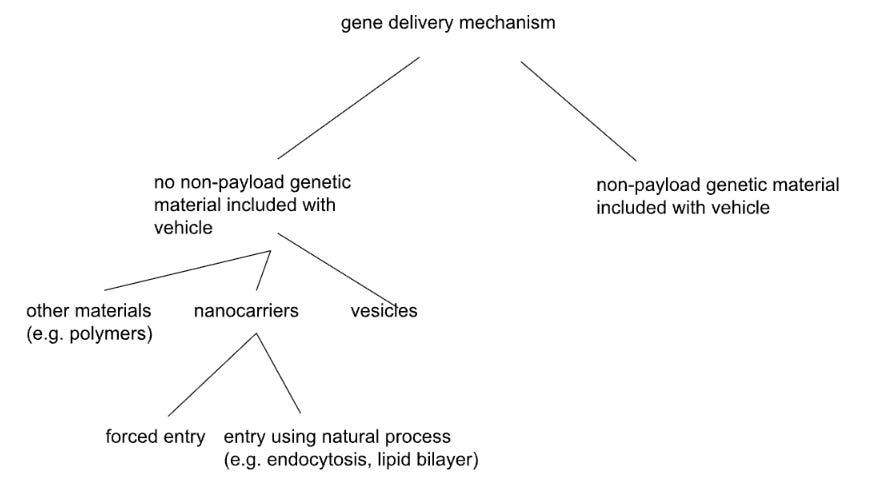
This was the first tiling tree we generated for the problem of gene delivery, and while it may seem passable, it actually has a few flaws that may prevent us from coming up with novel ideas.
Please note - the example we chose here may seem a bit technical. We will try to emphasize the logic that we applied for each decision, so don’t over worry about the technical details - although, if you want to learn more, there are lots of references you can identify (e.g., by searching Google Scholar, and so forth).
For example, our “delivery vehicle only” branch was split into vesicles, nanocarriers, and “other materials”. This was a retroactive split—we started by listing “vesicles” and “nanocarriers,” categories that we found already to exist in the literature. When you split by known solution types, the resulting tree might only produce variations within those categories. The structure itself could thus prevent you from finding anything that exists outside the taxonomy you’ve imported.
In addition, “other materials” is unsatisfying because it is a catch-all for many, potentially creative, ideas. But by not splitting out those “other materials,” we’ve converted a potential sea of opportunity into a dead end. It’s not just that the term is vague (though it is). It’s that vesicles and nanocarriers were interesting subsets, but there could be equally interesting alternatives that we have explicitly chosen not to identify. We simply left a catch-all bucket for whatever didn’t fit into the pre-existing categories - but there’s nothing to do next. You can’t explore systematically when the set you need to split into subsets is simply called “everything else.” What could the next split possibly be?
Compare that split to splitting by “number of kinds of molecule in the delivery vehicle”. This is a physical property that cuts across existing categories. A vesicle might contain one kind of component, or many, depending on its design. The mismatch between this split and the familiar-from-literature categories forces you to think about vesicles in new configurations. A single-component lipid-bound structure might behave extremely differently than a multi-component one - the latter might even approach the complexity of a simple living cell - such designs might not map cleanly onto “vesicle” at all. If your branches are named after solution subsets you could already Google, you could be just taxonomizing, not deconstructing in a fundamental way.
We also split by “forced entry” vs “natural process.” What does natural mean here? If you define it as “using endogenous cellular machinery,” then perhaps some viruses (despite being in nature) treat a cell naturally, and some treat the cell unnaturally. If you define it as “no exertion of physical force,” however, then you might consider a different set of viruses to use natural vs. unnatural processes. The word “natural” doesn’t map to a unique or specific physical property; it could mean different things in different contexts.
This vague categorization creates overlap between subsets split from a parent set. Consider endocytosis triggered by engineered ligands binding to receptors. The binding is artificial, but the internalization pathway is endogenous. Is this natural or forced? You can’t place a given solution without an arbitrary choice about which aspect matters more. Such terms are what we sometimes call “words that mean nothing” (WTMNs) - words that are not defined precisely enough to build conceptually on top of them. Of course, one can try to make a list of definitions of such words, and stick with them - that can greatly help, to have a “word choice list.” It can also be helpful to try to be more precise, reductionistic, or even quantitative, in one’s word choices.
Indeed, one fix, in the current example, is to use terms that reduce to atoms and bonds. Instead of forced vs natural, one might try “requires >X kJ/mol external energy input” where X might relate to some biophysically important threshold, or “disrupts lipid bilayer organization” (vs. not). These categories relate to measurements, not verbal interpretations. When you can’t classify a solution, it might be because your split is not a full, clean, mutually exclusive tiling, and that might be because you are using words that don’t have precise meanings.
The test: if replacing a word like “natural” with multiple other words that sound “natural”, but are mechanistically different, results in your solutions falling into different categories, then your original split may have been doing no work.
We tried again:
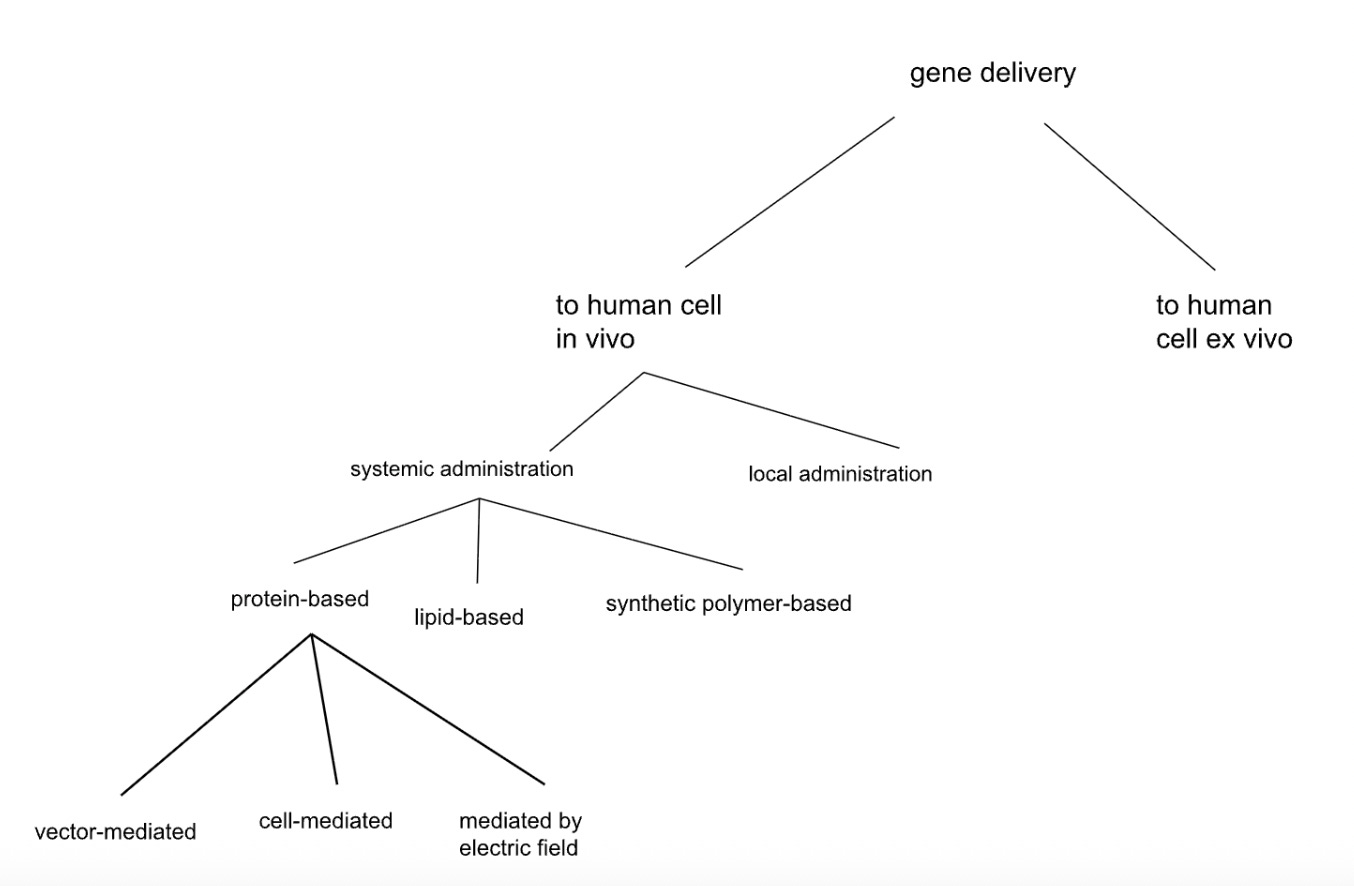
Second Tiling Tree
Our splits here, while more specific, still contained some vague phrasing and a considerable amount of overlap between branches, as well as splits that did not fully cover the space. For example, what does “based” mean in the context of “protein-based” or “lipid-based”? Can there be any protein in the lipid-based category? Is it a matter of percentages, and if so, is this an effective way to properly tile the space?
Tiling the space means that every possible solution must fall into exactly one category at each split. Like tiles on a floor—no gaps, no overlaps. When you split the delivery vehicles (e.g., those that are systemically delivered to the body, abbreviated “systemic” in the diagram) into “protein-based” and “lipid-based,” what about a vehicle that’s 50% protein and 50% lipid? It could fit both categories (overlap), or neither if you defined the categories as strictly containing one component (meaning many possibilities would be left off the table). The words do not have unique definitions. A proper tile might be “majority protein by mass” vs “not majority protein by mass”—now everything has exactly one home. The test: pick any random solution you can imagine. Can you find it by walking down your tree in exactly one path? If you hesitate at any branch, your tiles might be too fuzzy.
Additionally, look at the “protein-based” branch, which splits into “vector-mediated”, “cell-mediated”, and “field-assisted.” What do “mediated” and “assisted” actually mean, physically? And could something be both vector-mediated and field-assisted (e.g., an electric field could in principle get a virus across a barrier)? There is a bit of a category error here - the different subsets are of qualitatively different kinds. It’s like classifying vegetables as red, sweet, and crunchy - since the categories are along different dimensions, they inherently incur overlap.
A viral vector carrying a payload might fit the colloquial definition of vector-mediated. But the vector still has to interact with the cell membrane. Would that interaction count as cell-mediated? The three categories below “protein-based” are of different kinds (vector = payload carrier, cell = therapy recipient, field = physical mechanism) rather than being of the same kind (e.g., different types of protein-based carrier, or different ways of delivering a protein-based carrier). If we split “protein-based” (with the caveat, above, of its definitional ambiguity) into subsets of the same kind, then we reduce the risk of overlap. We are splitting along one consistent dimension.
Still, this tree generated zero new ideas because it’s organized around existing products. Every terminal node maps to something you could order from a catalog or find in a review paper. The splits encode what the field has done, not what the field could do.
Third Tiling Tree
Suppose we try a new split - say, by “living organism” vs “non-living assembly of multiple macromolecule types” vs “individual macromolecule.” Now, living organisms are subdivided into bacteria, yeast, mammalian cells, and so forth, according to standard taxonomy. Non-living assemblies might include liposomes, hydrogels, crystalline lattices, or even cork or cellulose structures. Individual macromolecules could be viral capsids, engineered proteins, or a strand of DNA that folds into origami.
That split works because the categories are quite distinct - they don’t overlap. (Indeed, as we write these words, we see - this could easily be two splits in a row - living vs. not, and then if not living, made of one thing or many.) The tree reveals gaps: we use mammalian cells, in therapeutics, but not nematodes. Why? Could nematodes have useful drug delivery properties or mechanisms? Curiously, a recent study proposes to use the toxoplasma parasite to deliver therapeutics to the brain2 - showing how vast the true space of possibility might be.
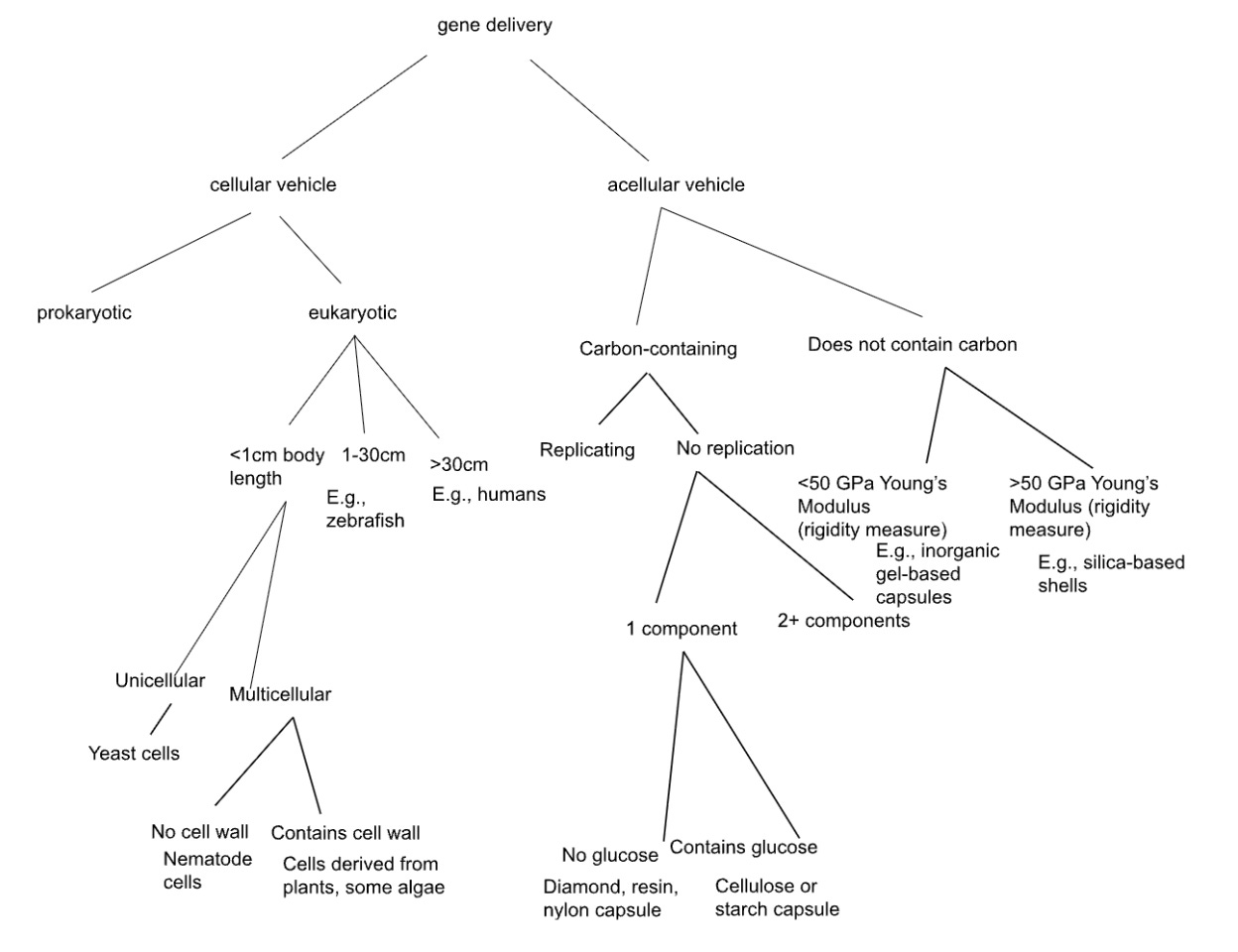
We thus created a final tree, using our insights from the first few iterations. We aimed to make every split as clearly defined as possible, using words with as precise a meaning as possible, and making the tiles as exclusive as possible. Using this tree, we came up with several new ideas (using yeast, nematode cells, and other novel ideas, for effective gene delivery).
Tree 3 attempts to split, whenever possible, by physical properties that don’t care about preexisting categories. The first split: cellular vs acellular (does the vehicle metabolize and replicate as a unit, or not). This forces you to consider whole organisms as delivery vehicles, not just biomolecular assemblies. Within cellular/eukaryotic, we split by organism body length: <1cm, 1-30cm, >30cm. Why? Because this determines practical lab handling, whether something would need to be introduced by surgery vs. bloodstream injection, and immune response scale.
Then, the uni- vs. multi-cellular sequence reveals a gap of possibility: we use bacteria (prokaryotic) and mammalian cells (large eukaryotic multicellular). But what about small eukaryotic unicellular organisms? That branch produces yeast—metabolically complex like mammalian cells, but with bacterial-scale ease of culturing and genetic manipulation. Yeast naturally produce vesicles and have well-characterized secretion pathways. Could yeast be engineered as a therapeutic?
The <1cm multicellular branch produces nematodes. C. elegans is 1 mm long - and, while a standard genetic model in biology, has never been (to our knowledge) used as a delivery vehicle itself. Could you engineer nematodes to migrate to target tissues and secrete therapeutic genes or gene products? Or some other organism, with the complexity of a nematode? The organism’s size makes it imageable in vivo, and its lifespan (weeks) matches many treatment timescales. Curiously, there is a field called “helminthic therapy” - which we only started to discuss after making this tree - in which parasitic worms are used to try to alter the immune system of human patients! Could such worms be used to deliver novel therapeutic agents too?
For acellular paths: “carbon-containing,” followed by “no replication”, followed by “1 component” raises the question - what single-component materials can encapsulate genes? If glucose-containing: perhaps cellulose or starch capsules. These are biocompatible, biodegradable, cheap, and can be chemically modified. On the carbonless branch, we split by a quantitative measure of material rigidity (Young’s modulus), another example of a strict and well-defined split.
In short, if you do not create the perfect tree to represent your problem space at first, do not fret. Spend some time critiquing your tree and thinking from first principles about whether your tree actually accomplishes the goal you set out for. From there, take all the time you need to retile your space and draw new trees. Repeat until you have an end product you’re satisfied with, and you’ll be on your way to solving some big problems!







Love this framing, this was a new way of breaking down tough problems. I've extended on this, writing my branches (splits) in dark red and my leaves (solutions) in blue. What do you make of that?